Click here to see the CDC’s latest vaccine guidelines for immunocompromised people and latest booster recommendations for others.
On August 12, the Food and Drug Administration (FDA) updated its emergency use authorization for the Pfizer-BioNTech and Moderna COVID-19 vaccines to allow for an additional dose for moderately to severely immunocompromised people.
“Today’s action allows doctors to boost immunity in certain immunocompromised individuals who need extra protection from COVID-19,” acting FDA commissioner Janet Woodcock, MD, said in a statement. “[O]ther individuals who are fully vaccinated are adequately protected and do not need an additional dose of COVID-19 vaccine at this time.”
UPDATE: On August 18, the Biden administration announced that, starting September 20, it would recommend a third Pfizer-BioNTech or Moderna shot for all adults eight months after receiving their second dose, pending FDA approval.
About 3% of the United States population—or about 7 million people—have weakened immunity due to medical conditions or the drugs used to treat them. But this group is at greater risk for severe COVID-19 and accounts for around 40% of people who are hospitalized for breakthrough SARS-CoV-2 infection after vaccination. Other countries, including France, Germany and Israel, already recommend additional vaccine doses for certain immunocompromised people, and the Centers for Disease Control and Prevention (CDC) estimates that around 1 million people in the United States have already received booster doses prior to authorization.
The following day, the CDC’s Advisory Committee on Immunization Practices (ACIP) voted unanimously in favor of boosters and specified which groups are eligible for an extra shot of the mRNA vaccines. The recommendation is based on studies showing that people with certain conditions and those taking certain medications do not respond as well to the vaccines and may benefit from a third dose.
Vaccination is key to helping us end the #COVID19 pandemic. Today, I signed ACIP recommendation endorsing use of an additional dose of COVID-19 vaccine for people with moderately to severely compromised immune systems after an initial two-dose series. https://t.co/QSwkXv4Cm5
— Rochelle Walensky, MD, MPH (@CDCDirector) August 13, 2021
This recommendation “is an important step in ensuring everyone, including those most vulnerable to COVID-19, can get as much protection as possible from COVID-19 vaccination,” said CDC director Rochelle Walensky, MD, MPH.
The CDC’s recommendation covers the following groups:
- People who have been receiving active cancer treatment for solid tumors or blood cancers;
- People who have received an organ transplant and are taking medication to suppress the immune system;
- People who have received a stem cell transplant within the last two years or are taking medication to suppress the immune system;
- People with moderate or severe primary immunodeficiency (conditions present from birth);
- People with advanced or untreated HIV;
- People taking high-dose corticosteroids or other medications that are immunosuppressive or immunomodulatory, including alkylating agents, antimetabolites, tumor necrosis factor blockers and certain other biologic agents.
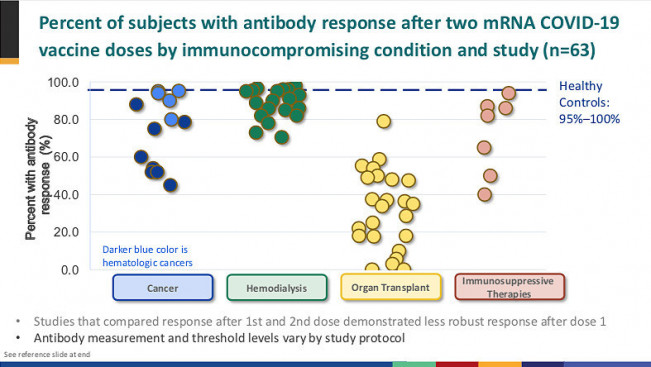
Organ transplant recipients generally take immune-suppressing medications to prevent rejection of the donor organ. Small studies have shown that liver, kidney and other transplant recipients have a weaker response to COVID-19 vaccines, but in many cases an extra dose can boost antibody levels.
Sometimes science works!
— Dorry Segev (@Dorry_Segev) August 13, 2021
3/15/21 we wrote first report (JAMA) that vaccines might be problem in transplant patients
5/5/21 we confirmed with first report (JAMA) of 2-dose regimen
6/14/21 we wrote first report (Annals) of benefit of third doses
8/13/21 FDA authorized 3rd doses!
“This is great news for the 7 million immunocompromised Americans, many of whom had no to little protective immune response to the standard one- and two-dose vaccine regimens and so faced risks similar to those who had chosen to take no vaccination at all, or worse, since COVID-19 infection raises our risks of severe effects from infection, including hospitalization and death,” Donna Cryer, president and CEO of the Global Liver Institute, said in a statement. She added that “transplant centers, cancer clinics and physician practices that treat the designated categories of immunocompromised patients must communicate with and schedule their patients for both antibody testing and vaccination.”
While several recent studies have shown that most people with solid tumors have a good antibody response after vaccination, those with blood (hematologic) cancers, such as leukemia or lymphoma, may not fare as well. A study from the Leukemia & Lymphoma Society (LLS) found that 25% of blood cancer patients do not produce an adequate response, but this varied widely by cancer type: Only 44% of people with mantle cell lymphoma had adequate antibody levels, rising to 100% for those with smoldering multiple myeloma or hairy cell leukemia. The type of cancer treatment is key to vaccine response. People who take monoclonal antibodies that target the CD20 receptor on antibody-producing B cells and those who use BTK inhibitors that impair B-cell function are least likely to respond. And the procedure used for CAR-T immunotherapy can wipe out all types of immune cells.
“LLS encourages anyone with a current or past blood cancer diagnosis who has already been vaccinated to talk with their health care team about whether and when they should receive another vaccine dose,” the organization states. “Those who are not vaccinated should consult with their health care team about starting the vaccination series as soon as possible.”
Some people with autoimmune conditions also take medications that interfere with immune response, including drugs that impair B-cell activity. Such individuals should consult their doctor to discuss whether they might benefit from a third vaccine dose. In some cases, medications can be delayed or temporarily stopped to give the immune system a chance to respond to the vaccines.
Courtesy of Centers for Disease Control and Prevention
Antibodies don’t tell the whole story, however. Antibody levels normally fall after natural infection or vaccination, but memory B cells can produce more as needed, and T-cell responses also play a role. While antibody tests are widely available, there is currently no simple and inexpensive test to determine broader immunity.
The situation is murkier for people living with HIV. Studies continue to yield conflicting evidence about whether HIV-positive people are more likely to develop severe COVID-19. Recent research has shown that about 75% of people with HIV show good immune response to natural SARS-CoV-2 infection, and small studies indicate that HIV-positive and HIV-negative people who received the Pfizer-BioNTech or Moderna mRNA vaccines had similar antibody and T-cell responses. But this may not be the case for those with more advanced HIV disease, unsuppressed viral load or low CD4 T-cell counts.
“People living with HIV are not a homogeneous group,” Monica Gandhi, MD, director of the Ward 86 HIV clinic at Zuckerberg San Francisco General Hospital, told POZ. “Many people living with HIV are doing very well on their antiretroviral therapy with high CD4 counts and suppressed viral loads. In general, we tend to boost vaccines when the CD4 count is below 200 as this indicates a more immunocompromised state.”
Responding to the CDC’s recommendation that people with advanced or untreated HIV are eligible for a third vaccine dose, Paul Sax, MD, of Brigham and Women’s Hospital in Boston said on Twitter, “People with untreated HIV need HIV treatment. They’d benefit way more from that than a COVID-19 vaccine booster.”
But advocates argue that all people with HIV should be eligible for a third dose, stating in an open letter to the CDC that restricting boosters to people with advanced or untreated HIV “is unnecessary, unreasonable and will result in logistical nightmares at vaccinations sites.”
“It’s ridiculous to try to codify ’advanced HIV.’ Today’s decision will just cause more confusion for people with HIV,” Lynda Dee, executive director of AIDS Action Baltimore, told POZ. “All people with HIV should have been included. That would have been clear and uncomplicated. People with HIV and their doctors should be able to decide together if a third vaccine dose is indicated.”
But the recommendation includes a clear statement that people should talk to their healthcare providers about their medical condition and whether getting an additional dose is appropriate for them, said Demetre Daskalakis, MD, MPH, director of the CDC’s Division of HIV/AIDS Prevention.
“The data we currently have really point toward folks who are more immunocompromised requiring an additional dose, as a safety net,” Daskalakis told POZ. “But people can have different levels of immunocompromise, and that’s something that can be discussed between a person in care and their physician.”
Immunocompromised people will not need a prescription from a doctor for a booster shot; they only have to attest that they have impaired immunity. The CDC advises that people should get a third dose of the vaccine they previously received, if possible. (The Pfizer-BioNTech vaccine is authorized for people ages 12 and older and the Moderna vaccine for those 18 and older.) The additional dose should be administered at least a month after the second shot. Although data are limited, an additional dose has no known risks, and side effects are similar to those seen with prior doses. The agency stressed that immunocompromised individuals should continue to take precautions, including wearing masks, social distancing and avoiding crowded indoor spaces, even after receiving an extra vaccine dose.
Anyone else surprised that today’s ACIP meeting didn’t recommend an mRNA booster for the vaccinated group of immunocompromised people that arguably might benefit the most? (I would recommend it.) @k_stephensonMD @CarlosdelRio7 @ENirenberg pic.twitter.com/Z2QU4QJk9v
— Paul Sax (@PaulSaxMD) August 13, 2021
The FDA authorization and CDC recommendation did not include immunocompromised people who received the single-shot Johnson & Johnson vaccine, due to a lack of data—a glaring omission as some research suggests that it provides a lower level of protection. The agencies said they are “actively engaged” in ensuring that people who received this vaccine have optimal protection.
The FDA stressed that people who are not immunocompromised do not need a booster shot at this time—an important consideration as vaccine supplies are limited and most people worldwide have yet to receive their first dose. But many experts expect that boosters may be needed eventually, especially for older people.
“The FDA is actively engaged in a science-based, rigorous process with our federal partners to consider whether an additional dose may be needed in the future,” Woodcock said.
Click here to read the FDA announcement.
Click here for the updated CDC recommendation.
Click here for more news about COVID-19 vaccines.







1 Comment
1 Comment