The high price of prescription medications is a hot topic these days. Many consumers are outraged about the amount they have to pay at the pharmacy and the Trump administration—as well as legislators on both sides of the aisle—have vowed to contain costs.
When it comes to Medicaid coverage, drugs that treat HIV and hepatitis C virus (HCV) have the dubious honor of being the greatest burden on health care expenditures compared with other categories of prescription drugs.
According to a recent analysis from Kaiser Family Foundation, of the $63.6 million that Medicaid spent on medications for outpatient treatment in 2017, 14 percent was for antiviral drugs. More than 90 percent of this category consisted of antiretrovirals (ARVs) to treat HIV and direct-acting antivirals (DAAs) to treat HCV. Antivirals were the No. 1 drug-class-expenditure category for Medicaid every year between 2014 and 2017.

Source: Kaiser Family Foundation
In 2017, Medicaid’s spending on antivirals included $5 billion for ARVs and $3.2 billion for DAAs. Since Gilead Sciences’ Sovaldi (sofosbuvir) hit the market in 2013, at the notorious cost of $1,000 per day—or $84,000 for a typical course of treatment—the price of DAAs has fallen steeply, thanks to increased competition by other pharmaceutical companies that now offer DAAs.

Source: Kaiser Family Foundation
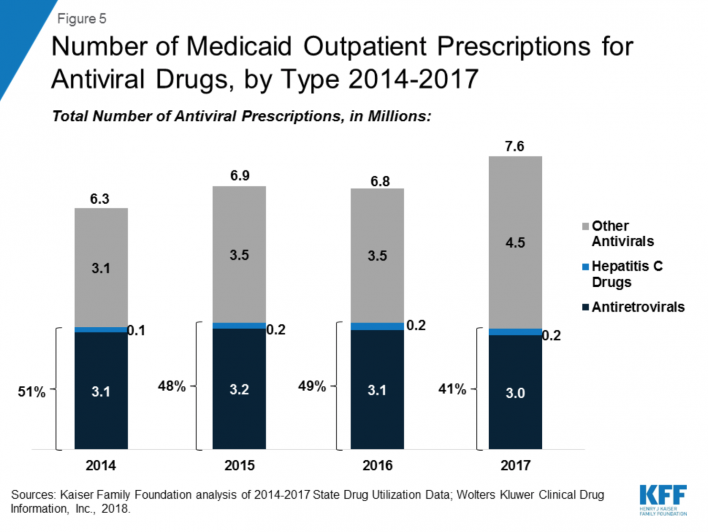
Source: Kaiser Family Foundation
Treatment for diabetes—a condition common among people with HIV—has become increasingly expensive in recent years as the cost of insulin has risen dramatically. Between 2014, when antidiabetic drugs placed only fifth in the rankings of Medicaid’s top drug-class expenditures, and 2017, when they placed second with a 10 percent share, Medicaid’s spending on this drug class doubled.
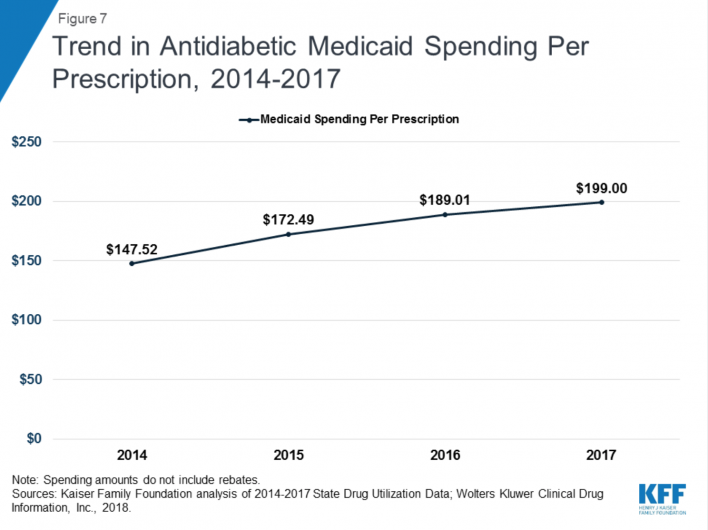
Source: Kaiser Family Foundation
How are Medications Priced?
The United States really doesn’t have laws governing drug prices, and the pharmaceutical industry has dodged various attempts to regulate these costs. This has resulted in the drug industry practice of “charging what the market can bear”—that is, “whatever we can get.” The high price of Sovaldi represented a major tipping point vis-à-vis the public’s tolerance for such tactics on the part of the highly profitable industry. There are, however, various ways that drugs are discounted as they make their way through the supply chain.
Branded Versus Generic Drugs
Medications are divided into two main categories. First, there are branded drugs that remain protected by a patent, which grants a single pharmaceutical company a monopoly on production, usually for for 20 years after a drug is invented. The clock typically starts ticking many years before the medication makes it through the clinical trials process and is approved. Consequently, pharmaceutical companies will often file multiple patents on the same drug in order to extend its patent life.
Then there are the generic drugs that have gone off patent, which allows competitors to enter the market and typically leads to lower prices. Some recent cases, however, have seen pharmaceutical companies engaging in price gouging on rarely used generic medications. The convicted felon “Pharma Bro” Martin Shkreli stoked public rage in 2015 when his company, Turing Pharmaceuticals, raised the price of Daraprim (pyrimethamine) from $13.50 to $750 per pill. Approved in the 1950s, Daraprim is indicated for the treatment of the parasitic infection toxoplasmosis, which is seen among people with advanced HIV disease, or AIDS.

Michael Halliday
Pricing
As a drug winds its way from the manufacturer to a wholesaler or direct purchaser, then to a pharmacy and finally to a consumer, its price is subject to a cascading series of adjustments.
The wholesale price is essentially the top price of a drug set by the manufacturer. Federal law defines the average manufacturer price, or wholesale acquisition cost, which is the average price at which the manufacturer actually sells the medication to wholesalers and community pharmacies. Such a price is shielded from the public but is known by federal officials, who use the figure to set various discounts for federal agencies (more on that below).
After purchasing pharmaceuticals from wholesalers, retailers typically mark up the price—known as the actual acquisition cost—by about 10 to 15 percent on brand-name drugs and to a higher extent on generics.
Insurers, meanwhile, use the muscle of pharmacy benefit managers (PBMs), which are often external companies, such as CVS Caremark, that work on behalf of multiple payers to negotiate discounts with pharmaceutical companies so that in the end insurance companies wind up with a smaller bill. Armed with the threat of adjusting their formularies to reduce or prevent access to a certain medication, PBMs also leverage the size of the entities they represent to reduce prices.
Cost Sharing For Consumers
When individuals fill their prescriptions at pharmacies (or perhaps through mail-order services), they are typically required to pay a share of the cost of the drug. (At that point the drug’s price is the actual acquisition cost plus the retailer’s markup.) Co-pays are a set fee established by the insurance company covering the medication and are often tiered as a way of encouraging consumers to opt for cheaper drugs. Generic drugs typically have the lowest co-pay, followed by branded drugs at a higher cost and the most expensive branded drugs in the top tier. Pricey ARVs and DAAs are typically in that top tier. A co-insurance, which is an alternative to a co-pay, requires the consumer to pay for a certain percentage of a medication’s cost.
Many insurance plans impose annual deductibles on consumers, requiring them to pay in full a certain amount of money each year before the insurer will start covering prescription drugs, medical care or both.
Co-Pay Assistance Cards
Various drug companies cover out-of-pocket costs—co-pays, co-insurance or deductibles—on behalf of people filling prescriptions. Consumers need only take the company’s co-pay assistance card to the pharmacy, which then handles all the accounting. Gilead, for example, offers up to $7,200 per year in cost-sharing coverage of Truvada (tenofovir disoproxil fumarate/emtricitabine) for pre-exposure prophylaxis (PrEP) with no monthly limit.

POZ
Some insurers don’t allow co-pay cards, however, or may not allow the cards to be applied toward an individual’s deductible. HIV advocates such as the AIDS Institute are going to battle to protect people living with and at risk for the virus from losing out on such a financial buffer.
Patient Assistance Programs
Pharmaceutical companies may have patient assistance programs, or PAPs, that provide their medications at no cost to low-income people who qualify.
340B Discount Program
Nonprofit community health centers have access to what’s known as the 340B discount program. This gives these clinics an automatic 23.1 percent discount off the average manufacturer price of a medication, and that discount is just a floor. After a drug is approved, whenever its manufacturer increases the price above the consumer price index (CPI)—the average annual increase in the cost of goods and services in the United States—the difference between the price increase and the CPI gets tacked on to the 340B discount. So if a drug cost has increased 6 percent in one year, but the CPI for that year was only 3 percent, that drug is then sold at an extra 3 percent discount to the community health centers.
Here’s the real kicker: Such health centers are allowed to charge insurance companies for the full price of these discounted drugs and spend the difference between the full and discounted drug price on their own programs.
According to President Trump’s 2020 budget request, the federal plan to control the HIV epidemic, designed by leaders at the Department of Health and Human Services and announced by the president at the February State of the Union address, would include $51 million annually to pay for PrEP through community health centers. If passed by Congress, presumably this dollar figure will be magnified through the 340B discount process. Such accounting magic will yield extra money to spend on promoting the use of PrEP as well as adherence to the regimen.
Medicare
Medicare Part D, a subset of the governmental insurance program for seniors and the disabled, covers prescription drugs. The legislation establishing this public benefit was passed in 2003 and, thanks to pharmaceutical industry lobbying, forbade the federal government from negotiating drug prices through Part D.
Antiretrovirals and some other types of medication belong to what’s known as a protected class of drugs according to Medicare Part D, which essentially means that all such medications must be on the program’s formulary.
The Trump administration is looking to combat high drug costs by placing new restrictions on these protected drug classes. For example, a physician may need first to prescribe a cheaper ARV—an increasing number of older HIV drugs have lost their patents—and then demonstrate that the drug failed before Medicare Part D will pay for a more expensive modern treatment. This process is known as step therapy. Additionally, prescriptions for drugs in the protected class might wind up triggering onerous prior authorization requirements.
Medicaid
Medicaid receives drug discounts according to the same formula as 340B. Reflecting the fact that people with HIV tend to have low incomes, Medicaid is the leading source of drug coverage for those receiving ARV treatment. An analysis of Medicaid spending in 2013 found that 280,000 HIV-positive individuals got their ARVs through the government insurance program. (An estimated 1.1 million people are living with HIV in the United States; about 690,000 of them are receiving medical care for the virus.)
This figure has likely grown in the years since, given that the expansion of state Medicaid programs under the Affordable Care Act (ACA, popularly known as Obamacare) brought many more people with the virus onto the public insurance program’s rolls. Currently, 14 states have not expanded Medicaid, including all Southern states with the exceptions of Arkansas and Louisiana. The five non-Southern states that have not expanded Medicaid are Kansas, Missouri, South Dakota, Wisconsin and Wyoming. The HIV epidemic is the most severe in the South, where half of all new infections occur.
ADAP
The AIDS Drug Assistance Program (ADAP) is a part of the Ryan White CARE Act. Passed in 1990 and reauthorized ever since, the act provides federal coverage for the care and treatment of low-income people with HIV. ADAP is a payer of last resort and will cover HIV treatment through a state-federal match system—and some states are much more generous than others—only if people cannot obtain coverage elsewhere. The expansion of state Medicaid programs has reduced the need for ADAP in recent years.
PrEP Pricing
The United States Preventive Services Task Force (USPSTF) has issued a draft proposal recommending PrEP as a grade-A prevention measure. If this proposal is adopted—which should be determined by this summer—insurance plans will have one year to cease imposing any cost sharing for PrEP or postexposure prophylaxis (PEP). That means no deductibles, co-pays or co-insurance for Truvada.
It is possible that insurers will wait until 2020 to notify their customers that their cost-sharing policy will change in 2021. So in at least some cases, the full benefit of this shift in policy may take nearly two years to go into effect.
Truvada is set to lose patent protection in 2021, at which point Teva Pharmaceuticals will likely have a six-month period of exclusive rights to produce a generic competitor. With only two companies producing the drug, the price may not fall a great deal during that short period. If Truvada goes off patent during the second half of 2021, this could mean that there will not be multiple companies producing generic versions until early-to-mid 2022. Once such competition begins, the price of the drug—currently about $1,600 to $2,000 per month—could possibly fall to as little as $30 per month.
A burgeoning and increasingly visible activist movement seeks to sidestep that three-year waiting period by pressuring the federal government to force Gilead to allow widespread generic manufacturing of Truvada immediately.

ACT UP member James Krellenstein, at a meeting at New York City’s LGBT Center protesting city STI clinic budget cuts, September 2015Benjamin Ryan
Meanwhile, Gilead will likely secure Food and Drug Administration approval for the use of Descovy (tenofovir alafenamide/emtricitabine) as PrEP by early 2020.
Compared with Truvada, Descovy was associated with improved markers of bone and kidney health among participants in a recent large study of both tablets’ use as PrEP. However, whether favoring Descovy over Truvada actually prevents bone fractures or kidney disease among HIV-negative individuals may remain a permanent open question. It is unlikely that studies will ever be conducted that will be long enough or large enough to observe a sufficient number of these relatively rare health outcomes from which to draw firm conclusion about the effects of Descovy versus Truvada. So once a generic version of Truvada is available—and that drug would be a bargain compared with Descovy—insurers may make it difficult for HIV-negative individuals to access the newer drug for prevention.







Comments
Comments